Current location: Home > NEWS > Corporate news
NEWS
PRODUCTS
Spacegen has once again passed the External Quality Evaluation with full score
News source: Release time:[2021-08-10]
Recently,the Clinical Laboratory Center (NCCL) from the National Health Commission announcedthe results of the “Bioinformatic Analysis of Laboratory Quality Evaluation from2021 National Tumor Somatic Mutation by High-throughput Sequencing”. The projectresults were announced by Spacegen Medical Laboratory (hereinafter referred toas Spacegen) that the results of Illumina, Ion Torrent, and BGI platforms from itslaboratory have all passed with full score. All the detection results wereconsistent with the standard, which once again has proved the bioinformatic analysisof Spacegen ‘s detection of tumor somatic mutations by high-throughputsequencing are professional and strict in quality control system.
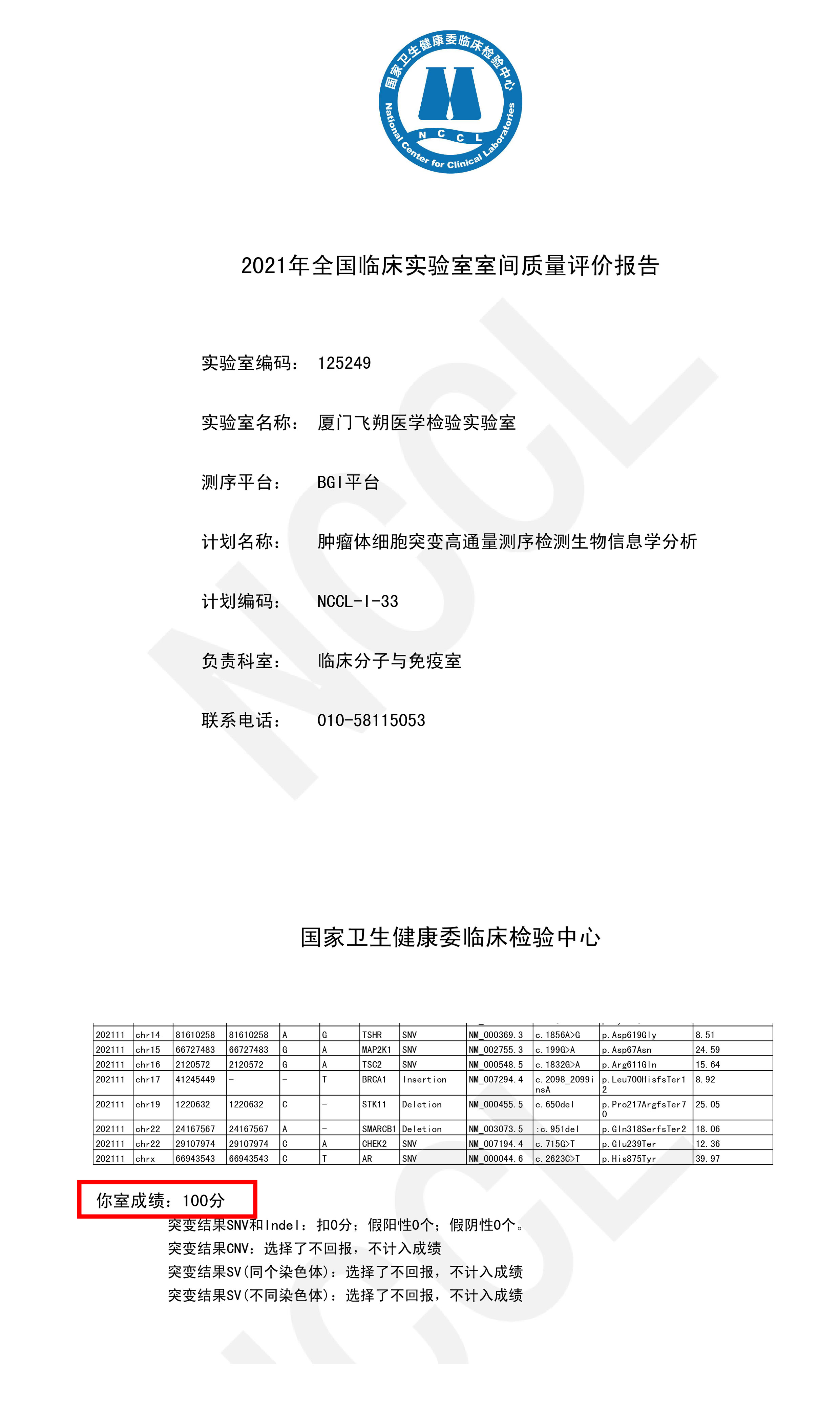


Thisexternal quality assessment is organized and implemented by the ClinicalLaboratory Center (NCCL) of the National Health Commission. It is an importantmeans to ensure the detection quality in clinical laboratories. It aims toassess the ability of bioinformatic analysis in tumor somatic gene mutations byclinical laboratories and institutions. At the same time, it finds problems ofbioinformatic analysis in the laboratories, which can help promoting thelaboratories to improve the bioinformatic analysis level.
SpacegenMedical Laboratory focuses on building a solid tumor center, which is committedto providing patients with tumor screening, diagnosis, drug guidance, prognosisevaluation, genetic tumor risk assessment and other overall solutions duringeach stage of disease progression. The laboratory has equipped with the IlluminaPlatform, ion torrent platform and other NGS platforms to provide patients withhigh quality and accurate detection services.